34. Why med-tech is the next big thing in India
Nitesh Kumar Jangir, co-founder of Innaccel tech, a Bangalore based tech company with the vision of giving quality healthcare access to everyone
Hello Kula community 👋
This week we are pleased to present to you our guest Nitesh Kumar Jangir, co-founder of Inaccel tech, Bangalore based tech company with the vision of giving quality healthcare access to everyone.
In our conversation, we discussed:
How did Nitesh discover the Medtech sectorWorking in medtech sector as an engineer vs as a doctorWhat is Innacel and what does it do in India and overseasR&D vs commercializations in the medtech industry: challenges and opportunitiesThe innovation and budget problem for India’s helthcare companiesMedtech market and trends
Now, buckle up for today’s post…
Key takeaways:
🛠️ Challenges and Opportunities in the Indian Market: India’s heavy reliance on imported medical devices underscores a significant gap in local innovation and manufacturing capabilities, despite government efforts to promote domestic production and innovation.
🫡 Regulatory and Commercialization Hurdles: Gaining global regulatory approvals and overcoming market entry barriers are crucial for medtech companies.
📈 Expansion and Market Penetration: As InnAccel expands into international markets, it highlights the strategic importance of addressing both domestic and global opportunities.
Who is Nitesh and why did he chose the engineering path?
Nitesh K. Jangir, an electronics engineer, has a diverse background across defense, industrial automation, and health technology.
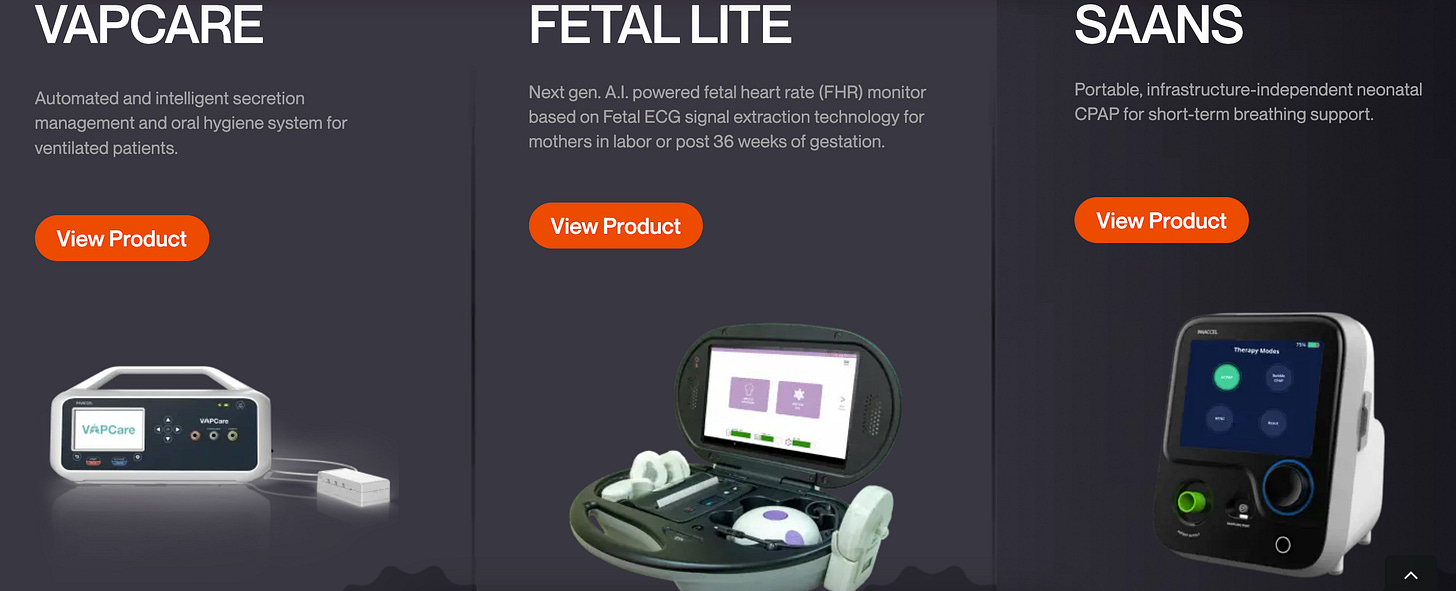
As a co-founder of Coeo Labs and InnAccel, he focuses on developing life-saving medical technologies, particularly in emergency and critical care, with an emphasis on maternal and child health. His innovations, including Saans and VAPCare, are implemented globally, improving healthcare outcomes and have obtained global certifications.
Nitesh's pioneering work has empowered caregivers to deliver critical interventions, impacting over 30,000 lives. Trained through the Stanford India Biodesign Program and the Chevening Fellowship at Oxford, he has filed and been granted sixteen patents in medical devices and AI.
Nitesh's contributions have earned him numerous accolades, including the Commonwealth Innovation Award, CES IFC Women Health Tech 2021, and recognition in Forbes 30 Under 30 for both India and Asia. He is also a Distinguished International Associate of the Royal Academy of Engineering UK and has received the National Technology Award from the Government of India.
Growing up in a small village in Rajasthan, where many lacked access to proper education, inspired my commitment to solving medical problems. I chose to pursue engineering instead of becoming a doctor because I wanted to empower healthcare professionals and have a greater impact. By developing new medical technologies, I aim to extend the reach and effectiveness of life-saving care, creating a multiplier effect that benefits more people - Nitesh
From supporting innovation to becoming a company - the story of Innacell
When Nitesh founded Coel Labs in 2014, a certified medical device company, solving unmet clinical needs in the field of emergency and critical needs, he had a very ambitious goal:
Use technology as a positive enabler for change in the medical sector in India and abroad
After several years of activity, more than 16 patents filled and millions of patients impacted, the goal has been widely achieved.
Coeo Labs gathered interest from Indian Medtech accelerator InnAccel, which saw potential in the company and decided to invest in its growth.In 2019, this partnership evolved into a merger between InnAccel and two of its incubatees, Coeo Labs and Sattva MedTech. Together, they formed a single entity focused on developing life-saving products that address critical medical challenges in emerging markets.
InnAccel has excelled in identifying critical unmet needs in healthcare and developing world-class products tailored to the resource constraints and price sensitivities unique to India.
As Nitesh said:
The focus remains on creating solutions designed specifically for the Indian market and its clinical needs—not just lower-cost versions of Western products, but innovations built from the ground up to meet the country's unique challenges.
R&D vs commercialization in the medtech industry and the impact of regulations
In the medical device industry, product development is crucial, but gaining global regulatory approval is equally important.
In India, international approvals, particularly from the USFDA, are often valued more than domestic certifications, making it essential for products to meet stringent international standards - Nitesh
For InnAccel, commercialization was initially challenging. They were introducing completely new products that doctors and hospitals were unfamiliar with, which meant they had to overcome several hurdles:
No clinical data
No pilot data
Certification gaps
This required answering more questions and putting in extra effort to convince stakeholders of their product's value. InnAccel had to not only sell their devices but also educate the market.
At Innaccel we effectively created a new product category and set a new price benchmark, avoiding early price wars and being an advange in commercialization - Nitesh
It wasn’t just about selling; it was about showing people who had a problem that they now had a solution.
As Nitesh rightly said:
InnAccel's innovations have the power to solve real challenges and, ultimately, save patients' lives.
India imports 75-80% of its medical devices - a problem of innovation or manufacturing capabilities?
India relies heavily on imported medical devices, with 75-85% of its medical device market, valued at $4.5 billion, being supplied by foreign countries.
Imports from the US, Germany, the Netherlands, and China continue to rise, but these products are typically optimized for the environments they were developed in, not for the Indian market - Nitesh
This creates a mismatch between the solutions available and the specific needs of India's healthcare system.
With the Indian government actively promoting MADE IN INDIA products and innovation, the goal is to drive the country toward self-reliance in medical technology, which still seems like a distant dream today with the import data continuing to grow.
Moreover, many Indian businesses due to the reduced cost of labour have grown into a “service mindset” and have difficulties to shift to a product mindset.
This can be observed when analyzing many large Indian corporations, that despite having the ability to invest in R&D they don’t consider it as their priority.
Indian medtech companies have an innovation problem
As highlighted by Nitesh during the interview, India still has a problem in the medical device industry. The challenge isn't just about manufacturing—it's about innovation.
One of the main issues is that doctors and engineers aren't trained to collaborate effectively, which hinders the development of new, impactful medical technologies. As a result, not much innovation is happening, and the products currently available are often not designed with India's unique needs in mind.
Another barrier to innovation in India's healthcare sector is the government's limited investment. Despite a modest increase in funding, healthcare still isn't a national priority. In FY23, India's investments in R&D were only about 1.58% of GDP, falling well short of the 2.5% target.
The 2024-2025 budget reflects this ongoing challenge. The Department of Health and Family Welfare's budget for FY 2024-25 stands at Rs 87,656.90 crores, a 12.93% increase from the previous year's Rs 77,624.79 crores. This rise aims to boost health services and infrastructure, but significant gaps remain in prioritizing healthcare at the national level.
Opportunities for foreign companies in India and government incentives
Since 2014 India allows 100% of FDI into the medical sector through direct route, aiming to boost foreign investments.
On top of that, the Government also implemented a series of PLI (Production Linked incentives) to promote local production of medical devices. However, this schemes have very high barriers to entry missing out on incentivizing the technology-driven startups or mid-sized Medtech companies in India that need funds for research and development.
Another key action that have been promoted is the formulation of 6 new strategies In November 2023, as part of National Medical Policy.
More on PLI here
More on India’s healthcare sector
Innacel expansion in Europe and the world
Innacel's products have received regulatory approval and are currently being utilized in leading hospitals in the US and UK. The company is now focusing on expanding into the German market.
While India represents a significant opportunity due to its large market, challenges such as difficult accessibility and the predominantly public healthcare system make it challenging to achieve substantial market penetration there.
For this reason international markets have been the priority of Innacel since day one!
***
To wrap up, Medtech market represent a huge opportunity in India, to innovate and create products tailored to its 1.3Bn population. Nitesh is a clear example of an Indian talent, that has decided to build its company in India to help Indians, offering a role model to follow for wannabe entrepreneurs and founders.
_________
Sources
eChai Ventures. (n.d.). Nitesh Kumar Jangir – Speaker profile. Retrieved January 27, 2025, from https://echai.ventures/speakers/nitesh-kumar-jangir
The New Indian Express. (2019, June 18). Indian engineer Nitesh Kumar Jangir wins innovation award in UK for neonatal breathing device. Retrieved January 27, 2025, from https://www.newindianexpress.com/world/2019/Jun/18/indian-engineer-nitesh-kumar-jangir-wins-innovation-award-in-uk-for-neonatal-breathing-device-1991978.html
Crunchbase. (n.d.). Nitesh Kumar Jangir. Retrieved January 27, 2025, from https://www.crunchbase.com/person/nitesh-kumar-jangir
European Union-India Chamber of Business and Industry (EICBI). (2022). 2022 Class of EuropeIndia40 leaders. Retrieved January 27, 2025, from https://www.eicbi.org/2022-class-europeindia40-leaders
India Briefing. (2020, November 13). India MedTech Industry Outlook: Growth and Opportunities. Retrieved January 27, 2025, from https://www.india-briefing.com/news/india-medtech-industry-outlook-growth-31918.html/
Moneycontrol. (2021, October 7). Technology has huge role in India’s healthcare, we aim to fill existing gaps: Siraj Dhanani, CEO, InnAccel. Retrieved January 27, 2025, from https://www.moneycontrol.com/news/trends/technology-has-huge-role-in-indias-healthcare-we-aim-to-fill-existing-gaps-siraj-dhanani-ceo-innaccel-8684971.html
YourStory. (2020, October 15). Funding alert: MedTech startup InnAccel raises funds from Mount Judi Ventures. Retrieved January 27, 2025, from https://yourstory.com/2020/10/funding-alert-medtech-startup-innaccel-mount-judi-ventures